
Chemistry, 18.01.2021 23:00 jeffljr2718
The titration of Na2CO3 with HCl has the following qualitative profile: a. Identify the major species in solution at points A-F. b. Calculate the pH at the halfway points to equivalence, B and D. [Key

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 00:00
Explain which group an element with the electron configuration 1s2 2s2 2p6 3s2 3p6 3d1 4s2 belongs to.
Answers: 3


Chemistry, 22.06.2019 07:30
All cells are made of four types of acids, lipids, proteins, and carbohydrates.
Answers: 1

Chemistry, 22.06.2019 10:00
Drug abuse will not lead to physical and psychological dependence. true or false ?
Answers: 2
You know the right answer?
The titration of Na2CO3 with HCl has the following qualitative profile: a. Identify the major specie...
Questions



Chemistry, 03.09.2021 19:10

Biology, 03.09.2021 19:10


Social Studies, 03.09.2021 19:10


Social Studies, 03.09.2021 19:10


History, 03.09.2021 19:10

Chemistry, 03.09.2021 19:10



English, 03.09.2021 19:10



Mathematics, 03.09.2021 19:10

English, 03.09.2021 19:10

Geography, 03.09.2021 19:10

Mathematics, 03.09.2021 19:10

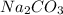
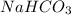
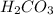
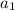 + log[
+ log[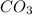
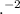 ]/[H
]/[H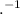 ]
]
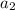 + log [H
+ log [H

