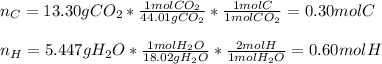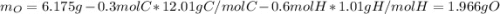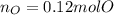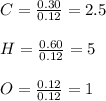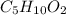Chemistry, 18.01.2021 21:40 5924000264
A 6.175 gram sample of an organic compound containing only C, H, and O is analyzed by combustion analysis and 13.30 g CO2 and 5.447 g H2O are produced. In a separate experiment, the molar mass is found to be 102.1 g/mol. Determine the empirical formula and the molecular formula of the organic compound.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 19:30
Which liquid (h2o, h2o + soap, or h2o + salt) has the strongest cohesion and adhesion? (need now plz)
Answers: 1

Chemistry, 23.06.2019 01:30
In which phase of mitosis do the spindle fibers pull the chromosomes apart to opposite sides of the cell ?
Answers: 1

Chemistry, 23.06.2019 04:31
Areaction is first order. if the initial reactant concentration is 0.0200 m, and 25.0 days later the concentration is 6.25 x 10-4 m, then its half-life is:
Answers: 1

You know the right answer?
A 6.175 gram sample of an organic compound containing only C, H, and O is analyzed by combustion ana...
Questions

Business, 13.10.2021 23:40


World Languages, 13.10.2021 23:40


Mathematics, 13.10.2021 23:40



Chemistry, 13.10.2021 23:40


Health, 13.10.2021 23:40

History, 13.10.2021 23:40




Mathematics, 13.10.2021 23:40

Physics, 13.10.2021 23:40



History, 13.10.2021 23:40