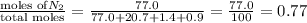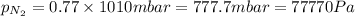Chemistry, 18.01.2021 21:10 jones501324
1. Consider the following properties of the atmosphere near the coast in Southern California: Average surface pressure: 1010 mbar Average temperature: 295 K Atmospheric gas composition (by volume): Nitrogen (N2) 77.0%, Oxygen (O2) 20.7%, Water (H2O) 1.4%, Argon (Ar), 0.9%. a. What is the average partial pressure of N2 in the atmosphere, pA, in units of Pa

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 23:00
Why are the trends and exceptions to the trends in ionization energy observed?
Answers: 1


Chemistry, 22.06.2019 10:30
Which of these is not an example of chemical weathering? a. iron-rich mineral rusting b. feldspar turning into clay c. limestone reacting with acid d. granite breaking up into sand
Answers: 1

Chemistry, 22.06.2019 10:30
Consider the following reactions. (note: (s) = solid, (l) = liquid, and (g) = gas.) mg(s) + ½o2(g) → mgo(s) + 146 kcal/mole h2(g) + ½o2(g) → h2o(g), δh = -57.82 kcal/mole what type of reaction is represented by the previous two examples?
Answers: 3
You know the right answer?
1. Consider the following properties of the atmosphere near the coast in Southern California: Averag...
Questions




Mathematics, 24.01.2021 21:50

Mathematics, 24.01.2021 21:50

Advanced Placement (AP), 24.01.2021 21:50

Computers and Technology, 24.01.2021 21:50

English, 24.01.2021 21:50

Chemistry, 24.01.2021 21:50

Mathematics, 24.01.2021 21:50


Chemistry, 24.01.2021 21:50



Mathematics, 24.01.2021 21:50



Mathematics, 24.01.2021 21:50

Mathematics, 24.01.2021 21:50

Mathematics, 24.01.2021 21:50

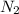 in the atmosphere is 77770 Pa.
in the atmosphere is 77770 Pa.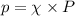
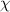 = mole fraction
= mole fraction 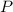 = total pressure
= total pressure