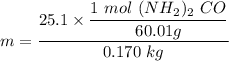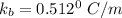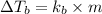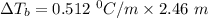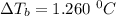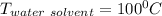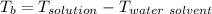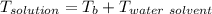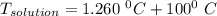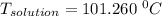Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 09:30
Which ocean zone has the most abundant primary producer and why a) the abyssopelagic zone ,du to the absence of light and cold water temperatureb) the bathypelagic zone, due to the absence of light and cold water temperaturec) the mesopelagic zone ,due to uts high light availability and warm water temperature d) the epipelagic zone,due to its high light availability and warm water temperature
Answers: 3


Chemistry, 22.06.2019 12:30
Suppose you wanted to make 100 grams of water. what is the molar mass of water (h2o)?
Answers: 2

Chemistry, 22.06.2019 23:30
Imagine a small synthetic vesicle made from pure phospholipids enclosing an interior lumen containing 1 mm glucose and 1 mm sodium chloride. if the vesicle is placed in pure water, which of the following happens faster? a. na+ diffuses out. b. cl– diffuses out. c. h2o diffuses in. d. glucose diffuses out. e. sodium chloride diffuses out.
Answers: 3
You know the right answer?
A solution is prepared by dissolving 25.1 g urea, (NH2)2CO, in 170.9 g water. Calculate the boiling...
Questions

Mathematics, 10.03.2021 14:00


Chemistry, 10.03.2021 14:00

Chemistry, 10.03.2021 14:00


English, 10.03.2021 14:00




Law, 10.03.2021 14:00



Mathematics, 10.03.2021 14:00

Mathematics, 10.03.2021 14:00

Spanish, 10.03.2021 14:00

Mathematics, 10.03.2021 14:00


Computers and Technology, 10.03.2021 14:00

Engineering, 10.03.2021 14:00

Mathematics, 10.03.2021 14:00