
Chemistry, 18.01.2021 18:10 mallorytaylor279
2.In a titration, 25.00 cm3 of a solution of hydrochloric acid reacted with 18.40 cm3 of sodium hydroxide solution of concentration 0.15 mol/dm3.
The equation which represents the reaction is:
HCl + NaOH > NaCl + H2O
Calculate the concentration of hydrochloric acid in mol/dm3

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 11:20
Sodium nitrite (nano2) reacted with 2−iodooctane to give a mixture of two constitutionally isomeric compounds of molecular formula c8h17no2 in a combined yield of 88%. draw reasonable structures for these two isomers. click the "draw structure" button to launch the drawing utility. place the two compounds in the appropriate boxes below.
Answers: 1


Chemistry, 22.06.2019 12:10
If a molecule with a molecular formula of c13h18 is treated with an excess of h2 in the presence of finally divided pt metal under conditions required for maximum hydrogenation of the molecule to give a molecule with a formula c13h24, how many rings are in the molecule?
Answers: 3

Chemistry, 22.06.2019 23:10
Afusion reaction takes place between carbon and another element. neutrons are released, and a different element is formed. the different element is a) lighter than helium.b)heavier than helium.c)the same weight as helium.d)dependent on the element that reacted with carbon.
Answers: 3
You know the right answer?
2.In a titration, 25.00 cm3 of a solution of hydrochloric acid reacted with 18.40 cm3 of sodium hydr...
Questions

Geography, 09.10.2019 22:00

World Languages, 09.10.2019 22:00

Computers and Technology, 09.10.2019 22:00



Physics, 09.10.2019 22:00


Biology, 09.10.2019 22:00



English, 09.10.2019 22:00








Mathematics, 09.10.2019 22:00

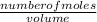 =
= 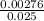 = 0.11mol/dm³
= 0.11mol/dm³

