
Chemistry, 18.01.2021 16:50 dragongacha777
PLEASE HELP ME
1. What is the molality of a solution made up of 43.6 mol of CaCl2 dissolved by 13.5 kg of water?
2. How many moles of Na2CO3 are required to create 9.54 liters of a 3.4 M solution?
3. Which of these actions can result in decreasing the molarity of a solution?
Select all that apply.
A
adding solute
B
adding solvent
C
removing solute
D
removing solvent
Thanks Y'all:)

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 17:40
Areaction in which products can react to re-form reactants is
Answers: 1



Chemistry, 23.06.2019 00:00
What is the empirical formula of a compound that is 50.7% antimony and 49.3% selenium ?
Answers: 2
You know the right answer?
PLEASE HELP ME
1. What is the molality of a solution made up of 43.6 mol of CaCl2 dissolved by 13.5...
Questions


Social Studies, 11.11.2020 22:10


Physics, 11.11.2020 22:10


Mathematics, 11.11.2020 22:10


Mathematics, 11.11.2020 22:10



History, 11.11.2020 22:10

Biology, 11.11.2020 22:10

Mathematics, 11.11.2020 22:10

Biology, 11.11.2020 22:10

English, 11.11.2020 22:10

Social Studies, 11.11.2020 22:10




Mathematics, 11.11.2020 22:10

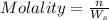
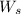 = weight of solvent
= weight of solvent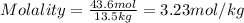
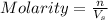
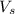 = volume of solution in L
= volume of solution in L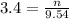
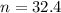
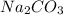 is 32.4
is 32.4 

