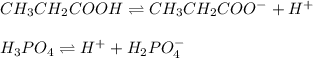Chemistry, 18.01.2021 14:00 zalyndevola
Propionic acid (CH3CH-COOH) has a K, 1.3 x 10^-5 and phosphoric acid (H3PO2) has a Ka = 7.5 x 10^-3
Choose the conjugate base for each.
A. CH3CH2COO2- for CH3CH2COOH; HPO4 2- for H3PO4
B. CH3CH2CO- for CH3CH2COOH; H2PO3 - for H3PO4
C. CH3CH2COOH2 for CH3CH2COOH; H4PO4 for H3PO4
D. CH3CH2COO- for CH3CH2COOH; H2PO4 - for H3PO4

Answers: 1


Another question on Chemistry

Chemistry, 23.06.2019 15:50
Astable atom that has a large nucleus most likely contains 1. more neutrons than protons. 2.more protons than neutrons. 3.equal numbers of protons and neutrons. 4.changing numbers of protons and neutrons.
Answers: 2

Chemistry, 23.06.2019 17:50
The only bonds in a formula unit of caf2 are nonpolar covalent polar covalent ionic metallic
Answers: 1

Chemistry, 23.06.2019 18:30
Why must pencil be used to draw on paper in paper chromatography
Answers: 2

Chemistry, 23.06.2019 21:00
Using the periodic table choose the more reactive nonmetal s or as
Answers: 1
You know the right answer?
Propionic acid (CH3CH-COOH) has a K, 1.3 x 10^-5 and phosphoric acid (H3PO2) has a Ka = 7.5 x 10^-3...
Questions

Mathematics, 05.03.2021 22:40


Computers and Technology, 05.03.2021 22:40


Mathematics, 05.03.2021 22:40

Mathematics, 05.03.2021 22:40

Mathematics, 05.03.2021 22:40




History, 05.03.2021 22:40


Mathematics, 05.03.2021 22:40


Mathematics, 05.03.2021 22:40


History, 05.03.2021 22:40

Mathematics, 05.03.2021 22:40

Chemistry, 05.03.2021 22:40