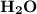Chemistry, 17.01.2021 18:40 richlovedarkwa5
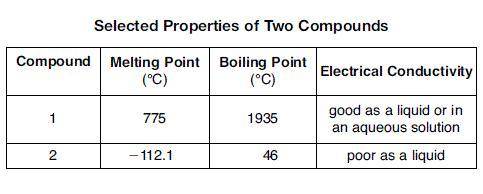
The table below shows properties of two compounds at standard pressure. Which statement classifies the two compounds? 1)Both compounds are ionic, 2)Both compounds are molecular, 3) Compound 1 is ionic, and compound 2 is molecular, 4)Compound 1 is molecular, and compound is ionic

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 18:00
Mercury turns to vapor at 629.88 k how much heat is lost 175 g of mercury vapor at 650 current condenses to a liquid at 297 ca mercury turns to weber at 629.88 kelvin how much he is lost 175 g of mercury vapor and 650 coming condensers to liquidate 297 kevin
Answers: 2


Chemistry, 22.06.2019 20:30
We are hoping to create 5.72 grams of glucose. the plant was given 4.75 liters of co2 and 2.81 g of h20. which reactant was the limiting reagent? how much excess mass did we have of the other reactant?
Answers: 2

Chemistry, 22.06.2019 22:00
Scientists often have to deal with numbers that are either very large or very small. for example, the radius of the sun is approximately 696,000 kilometers, while bacterial cells are as small as 1.9 × 10-4 millimeters. express each number in an alternate form.
Answers: 1
You know the right answer?
The table below shows properties of two compounds at standard pressure. Which statement classifies t...
Questions


Biology, 25.07.2019 21:00




Social Studies, 25.07.2019 21:00

History, 25.07.2019 21:00










Social Studies, 25.07.2019 21:00

Mathematics, 25.07.2019 21:00

Chemistry, 25.07.2019 21:00