
Chemistry, 17.01.2021 18:40 paulitaaustin
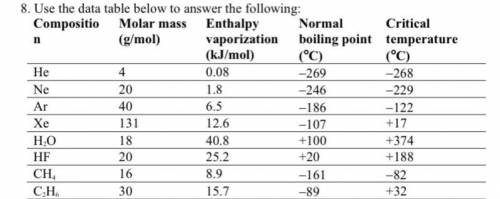
A. Among nonpolar liquids, those with higher molar masses tend to have normal boiling points that are (higher, lower, or about the same).
b. Among compounds of approximately the same molar mass, those with greater polarities tend to have enthalpies of vaporization that are (higher, lower, or about the same).
c. Which is the only noble gas listed that is stable as a liquid at 0°C? Explain your answer using the concept of critical temperature.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 12:30
Which statement is true about this reaction? 14n+1h 15o it is a practical source of energy on earth. it occurs only outside the solar system. its product is heavier than each of its reactants. it shows the critical mass of an element.
Answers: 2

Chemistry, 22.06.2019 14:40
Pastoral farming is best described as a. a method of raising livestock and moving herds b. an african method of agriculture c. a method of cultivating crops on poor soils d. a common method of desert farming select the best answer from the choices provided a b c d
Answers: 2

Chemistry, 22.06.2019 19:30
If 16.00g of hydrogen gas reacts with 126.73g of oxygen, how many grams of water are yielded? (both reactants are completely consumed in the reaction.)
Answers: 2

Chemistry, 22.06.2019 22:00
The volume of an unknown substance in a sealed glass jar is 50 milliliters. the volume of the jar is 200 milliliters. which state of matter could the substance be?
Answers: 2
You know the right answer?
A. Among nonpolar liquids, those with higher molar masses tend to have normal boiling points that ar...
Questions

Mathematics, 17.09.2020 20:01

Mathematics, 17.09.2020 20:01

Biology, 17.09.2020 20:01

Mathematics, 17.09.2020 20:01

Mathematics, 17.09.2020 20:01

Mathematics, 17.09.2020 20:01

Mathematics, 17.09.2020 20:01

Mathematics, 17.09.2020 20:01

Mathematics, 17.09.2020 20:01

Mathematics, 17.09.2020 20:01

Social Studies, 17.09.2020 20:01

Mathematics, 17.09.2020 20:01

Mathematics, 17.09.2020 20:01

Mathematics, 17.09.2020 20:01

Mathematics, 17.09.2020 20:01

Mathematics, 17.09.2020 20:01

Mathematics, 17.09.2020 20:01

Mathematics, 17.09.2020 21:01

Mathematics, 17.09.2020 21:01

Chemistry, 17.09.2020 21:01



