
Chemistry, 17.01.2021 16:10 rogersdeloris1ovgm3b
calculate the freezing point of 3.46 gram of a compound X in 160 gram of benzene when a separate sample of X was vaporized it's density was found to be 3.27 gram/liter at 116°c and 773 torr. The freezing point of pure benzene is 5.45°c of Kf=5.1°/m

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 20:10
Insoluble sulfide compounds are generally black in color. which of the following combinations could yield a black precipitate? check all that apply. na2s(aq)+kcl(aq) li2s(aq)+pb(no3)2(aq) pb(clo3)2(aq)+nano3(aq) agno3(aq)+kcl(aq) k2s(aq)+sn(no3)4(aq)
Answers: 1

Chemistry, 22.06.2019 21:50
28. which is not a reason that water is used to store spent fuel rods from nuclear power plants? water increases the speed of the chain reaction in the fuel rods. water protects nuclear power plant workers from the high temperature and radiation of the fuel rods. water acts as a radiation shield to reduce the radiation levels. water cools the spent rods. salts action
Answers: 1

Chemistry, 22.06.2019 22:00
Ill give u brainliest pls how is mass of carbon conserved during cellular respiration
Answers: 1

You know the right answer?
calculate the freezing point of 3.46 gram of a compound X in 160 gram of benzene when a separate sam...
Questions


History, 27.02.2020 18:33



History, 27.02.2020 18:34

Mathematics, 27.02.2020 18:34

Arts, 27.02.2020 18:34




Mathematics, 27.02.2020 18:34


Engineering, 27.02.2020 18:34





Mathematics, 27.02.2020 18:34








 = depression in freezing point =
= depression in freezing point =  =
= 
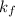 = freezing point constant = 5.1
= freezing point constant = 5.1 




