
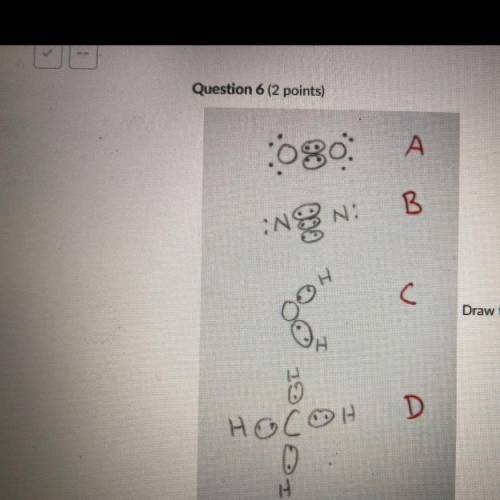
Draw the Lewis structures of N2, O2, H20, and CH4. Compare your drawing to the ones in the drawing on this test and select
the answer that best describes which drawing is wrong and why. Note I drew circles
around electrons that are participating in covalent bonding. This is normally not done
but for the purpose of this test the circled electrons are fine.
A: O2 Is wrong because it shows the electrons at a 45 degree angle to the
Oxygen atoms.
B: N2 is wrong because it shows a triple bond.
C: H2O is wrong because it is missing 4 valence electrons.
D: CH4 is wrong because the bonds are supposed to be bent at 109.5 degrees.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 07:30
Label a-f based on the table using c for concentrated and d for dilute
Answers: 2

Chemistry, 22.06.2019 12:20
Which is an example of the practical pursuit of alchemy? a. forming perfect substances. b. transforming base metals. c. developing metalworking techniques. d. linking spiritual characteristics with material substances.
Answers: 1

Chemistry, 22.06.2019 20:30
We are hoping to create 5.72 grams of glucose. the plant was given 4.75 liters of co2 and 2.81 g of h20. which reactant was the limiting reagent? how much excess mass did we have of the other reactant?
Answers: 3

Chemistry, 23.06.2019 09:00
How many grams of ammonia are produced when 1.0 mole of nitrogen reacts
Answers: 2
You know the right answer?
Draw the Lewis structures of N2, O2, H20, and CH4. Compare your drawing to the ones in the drawing o...
Questions


Mathematics, 27.04.2021 03:40

English, 27.04.2021 03:40



Mathematics, 27.04.2021 03:40

Business, 27.04.2021 03:40





Chemistry, 27.04.2021 03:40



Mathematics, 27.04.2021 03:40

Biology, 27.04.2021 03:40


Computers and Technology, 27.04.2021 03:40

Chemistry, 27.04.2021 03:40

Chemistry, 27.04.2021 03:40



