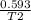Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 10:20
In a reaction equation, where are the products located? a.) above the arrow b.) to the right of the arrow c.) to the left of the arrow d.) below the arrow
Answers: 2

Chemistry, 22.06.2019 14:30
Calculate the mass of carbon in 97.0 g of sucrose c12h22o11
Answers: 3

Chemistry, 22.06.2019 21:30
How many oxygen atoms are there in 3.15 moles of hcl manganese (iv) oxide, mno2
Answers: 2

Chemistry, 23.06.2019 00:00
2-bromo-2-methylbutane undergoes an e1 elimination reaction in the presence of ethanol. in the next reaction only one of the possible products is represented. although the product shown is not the major product of the reaction, notice that there is more than one way it can be produced. complete the mechanism and draw the missing substances.
Answers: 1
You know the right answer?
a sample of methane gas at a pressure of 0.815 atm and a temperature of 265 C occupies a volume of 4...
Questions

English, 22.10.2020 06:01

Social Studies, 22.10.2020 06:01

Mathematics, 22.10.2020 06:01

History, 22.10.2020 06:01




Computers and Technology, 22.10.2020 06:01


Advanced Placement (AP), 22.10.2020 06:01

Biology, 22.10.2020 06:01


Social Studies, 22.10.2020 06:01

Biology, 22.10.2020 06:01



Mathematics, 22.10.2020 06:01


Biology, 22.10.2020 06:01

History, 22.10.2020 06:01

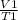 =
= 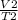
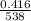 =
=