
Chemistry, 16.01.2021 23:40 dewaynesmith46
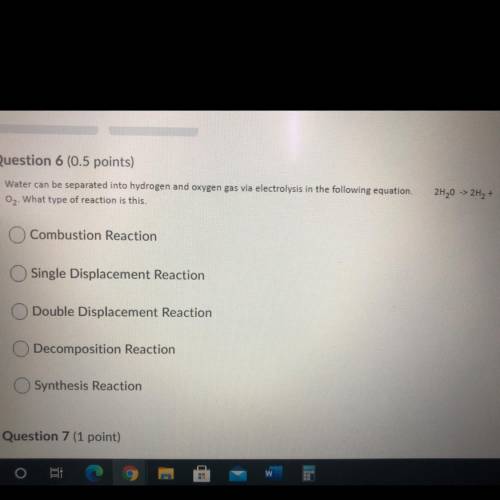
Water can be separated into hydrogen and oxygen gas via electrolysis in the following equation. 2H20 -> 2H2 +O2 What type of reaction is this.

Answers: 3


Another question on Chemistry


Chemistry, 22.06.2019 19:30
If 16.00g of hydrogen gas reacts with 126.73g of oxygen, how many grams of water are yielded? (both reactants are completely consumed in the reaction.)
Answers: 2

Chemistry, 22.06.2019 21:00
The rate constant for the reaction below is 6.2 x 10−5 mol l−1 s −1. if the initial concentration of a is 0.0500 m, what is its concentration after 115 s?
Answers: 1

Chemistry, 22.06.2019 21:00
Which answer tells the reason the earth’s climate is getting warmer? too many animals are becoming extinct. large glaciers are melting in antarctica. the earth is moving closer to the sun. driving cars gives off gases that trap heat in the atmosphere.
Answers: 1
You know the right answer?
Water can be separated into hydrogen and oxygen gas via electrolysis in the following equation. 2H20...
Questions


English, 16.04.2021 23:10


Mathematics, 16.04.2021 23:10

English, 16.04.2021 23:10

Advanced Placement (AP), 16.04.2021 23:10

Mathematics, 16.04.2021 23:10






Mathematics, 16.04.2021 23:10

Mathematics, 16.04.2021 23:10






Mathematics, 16.04.2021 23:10



