Data
Record your data either in your lab notebook or in the space below.
Aluminum
Coppe...

Chemistry, 15.01.2021 18:20 sciencefanfae7248
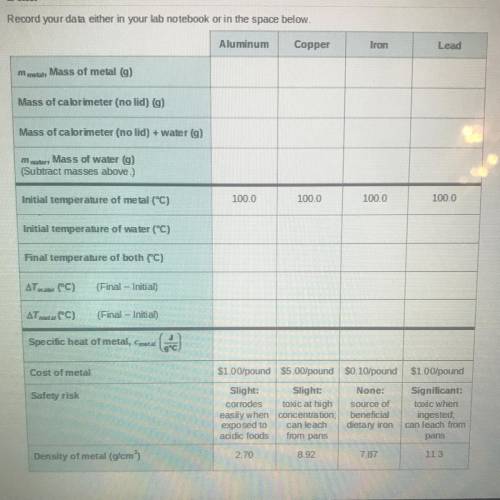
Data
Record your data either in your lab notebook or in the space below.
Aluminum
Copper
Iron
Lead
m
mobile
Mass of metal g)
Mass of calorimeter (no lid) g)
Mass of calorimeter (no lid) + water (g)
m
Mass of water g)
(Subtract masses above.)
100.0
100.0
100.0
Initial temperature of metal (°C)
100.0
Initial temperature of water (°C)
Final temperature of both (°C)
AT water (C)
(Final - Initial)
ATmetal (°C)
(Final - Initial)
Specific heat of metal, Cmetal

Answers: 2


Another question on Chemistry


Chemistry, 22.06.2019 11:00
An object becomes electrically charged when: electrons are created in it electrons from it are destroyed electrons are transferred to it protons from it are destroyed protons are created in it
Answers: 1

Chemistry, 22.06.2019 22:00
Choose all the answers that apply. fluorine (f) has an atomic number of 9 and an atomic weight of 18.99. fluorine has a. 9 protons b. 10 neutrons c. 18 electrons d. an atomic mass of 19 e. at least one isotope
Answers: 1

Chemistry, 23.06.2019 00:30
What is calcium oxide+diphosphorus pentoxide--> calcium phosphate balanced
Answers: 1
You know the right answer?
Questions



English, 07.12.2020 23:20

Mathematics, 07.12.2020 23:20


English, 07.12.2020 23:20

Mathematics, 07.12.2020 23:20

Computers and Technology, 07.12.2020 23:20

Spanish, 07.12.2020 23:20

Mathematics, 07.12.2020 23:20


Physics, 07.12.2020 23:20

Mathematics, 07.12.2020 23:20


Mathematics, 07.12.2020 23:20




Arts, 07.12.2020 23:20



