Cynthia has 200kg of magnesium. Calculate how much MgCl2 she can make.
Mg + Cl2 = MgCl2...


Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 20:30
If 10.g of agno3 is available, what volume of 0.25 m agno3 can be prepared
Answers: 1

Chemistry, 22.06.2019 07:00
Achemist wants to extract copper metal from copper chloride solution. the chemist places 0.50 grams of aluminum foil in a solution containing 0.75 grams of copper (ii) chloride. a single replacement reaction takes place. (ii) chloride. a single replacement reaction takes place. which statement explains the maximum amount of copper that the chemist can extract using this reaction? a) approximately 0.36 grams, because copper (ii) chloride acts as a limiting reactant b) approximately 1.8 grams, because copper (ii) chloride acts as a limiting reactant c) approximately 0.36 grams, because aluminum acts as a limiting reactant d) approximately 1.8 grams, because aluminum acts as a limiting reactant
Answers: 3

Chemistry, 22.06.2019 09:10
Select the correct answer from each drop-down menu.describe what happens to a carbon-11 atom when it undergoes positron emission.the decay of a carbon-11 atom _1_, and this causes it to emit _2_.options for 1: > changes a neutron into a proton> changes a proton into a neutron> is hit with a neutron> reconfigures its protons and neutronsoptions for 2: > a negatively charged electron-sized particle> a positively charged election-sized particle> two atoms and several neutrons> two neutrons and two protons
Answers: 3

Chemistry, 22.06.2019 12:00
Marcel just purchased 1.69 grams of iron fillings in order to make living putty for his 6 year old niece. how many moles of iron are made in his sample?
Answers: 1
You know the right answer?
Questions

Mathematics, 02.11.2020 18:30

English, 02.11.2020 18:30


Biology, 02.11.2020 18:30



Chemistry, 02.11.2020 18:30





History, 02.11.2020 18:30


Chemistry, 02.11.2020 18:30

English, 02.11.2020 18:30

Mathematics, 02.11.2020 18:30

English, 02.11.2020 18:30

English, 02.11.2020 18:30

English, 02.11.2020 18:30

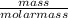 =
= 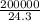 = 8230.5mole
= 8230.5mole 

