

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 05:30
What type of reaction is shown below? check all that apply. 2h2o2 → 2h2o + o2 synthesis decomposition combustion
Answers: 3

Chemistry, 22.06.2019 07:00
Indicate whether the specified alkyl halides will form primarily substitution products, only elimination products, both substitution and elimination products, or no products when they react with sodium methoxide. 1-bromobutane 1-bromo-2-methylpropane 2-bromobutane 2-bromo-2-methylpropane
Answers: 2

Chemistry, 22.06.2019 13:00
16. why must the number of electrons lost equal the number of electrons gained in every redox reaction? use 3 – 4 sentences in your own words to address this question. 18. what type of radiation is emitted when chromium-51 decays into manganese-51? show the nuclear equation that leads you to this answer. 19. a radioactive nucleus alpha decays to yield a sodium-24 nucleus in 14.8 hours. what was the identity of the original nucleus? show the nuclear equation that leads you to this answer.
Answers: 2

Chemistry, 22.06.2019 13:50
Amap that uses a range of colors and shading to represent the elevation, depth, or landscape of specific features on earth is a/an map.
Answers: 3
You know the right answer?
4. Calculate the molar mass of Fe2(SO4)3. Then calculate the mass of iron(III) sulfate of 4.05X1023...
Questions



Mathematics, 15.10.2020 07:01


Biology, 15.10.2020 07:01

Mathematics, 15.10.2020 07:01





Chemistry, 15.10.2020 07:01

Mathematics, 15.10.2020 07:01

Mathematics, 15.10.2020 07:01


Mathematics, 15.10.2020 07:01

Computers and Technology, 15.10.2020 07:01


Mathematics, 15.10.2020 07:01

Mathematics, 15.10.2020 07:01

English, 15.10.2020 07:01

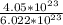 =269
=269

