
Look at the following enthalpy diagram. Select all that apply.
A.)The products have more energy than the reactants.
B.)This is an addition reaction.
C.)A large activation energy is required for this reaction to take place.
D.)The products are more stable than the reactants.
E.)This is a substitution reaction.
You may have more than one answer.
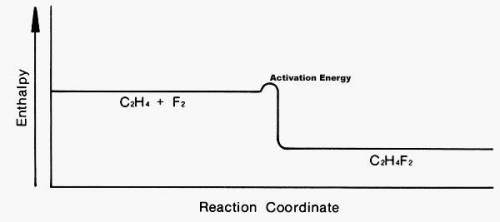

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 05:30
Compare and contrast physical changes with chemical changes.
Answers: 1

Chemistry, 22.06.2019 09:30
The chart shows the bid provided by four contractors to complete a job. which contractor is the most cost-effective?
Answers: 3

Chemistry, 22.06.2019 16:30
Ammonium perchlorate nh4clo4 is the solid rocket fuel used by the u.s. space shuttle. it reacts with itself to produce nitrogen gas n2 , chlorine gas cl2 , oxygen gas o2 , water h2o , and a great deal of energy. what mass of nitrogen gas is produced by the reaction of 2.1g of ammonium perchlorate?
Answers: 2

Chemistry, 23.06.2019 03:00
Abaker touches a pie right after taking it out of the oven. which statement best explains why the pie feels hot?
Answers: 2
You know the right answer?
Look at the following enthalpy diagram. Select all that apply.
A.)The products have more energy tha...
Questions


Chemistry, 17.08.2021 03:20

Mathematics, 17.08.2021 03:20


History, 17.08.2021 03:20

Computers and Technology, 17.08.2021 03:20


English, 17.08.2021 03:20


Mathematics, 17.08.2021 03:20

Mathematics, 17.08.2021 03:20


Mathematics, 17.08.2021 03:20

Health, 17.08.2021 03:20

English, 17.08.2021 03:20

Physics, 17.08.2021 03:20

Social Studies, 17.08.2021 03:20


Mathematics, 17.08.2021 03:20

Biology, 17.08.2021 03:20



