
Chemistry, 14.01.2021 19:10 FailingstudentXD
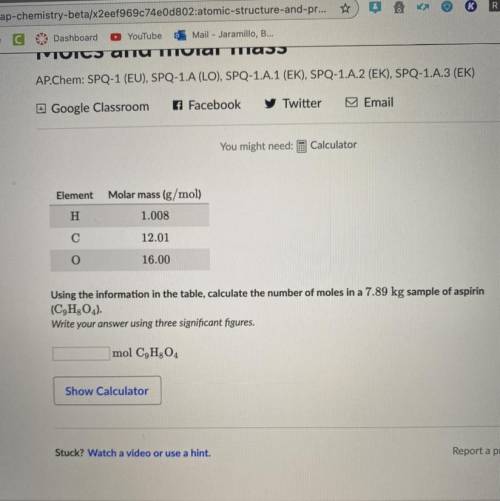
Element
Molar mass (g/mol)
H
1.008
С
12.01
O
16.00
Using the information in the table, calculate the number of moles in a 7.89 kg sample of aspirin (C9H2O4).
Write your answer using three significant figures.
mol C9H304
Show Calculator

Answers: 3


Another question on Chemistry


Chemistry, 22.06.2019 09:00
What type of energy do chemical bonds have? what type of energy is it converted to during chemical reactions? question 15 options: chemical bonds have kinetic energy, which is converted to potential energy during chemical reactions. chemical bonds have electric energy, which is converted to potential energy during chemical reactions. chemical bonds have heat energy, which is converted to kinetic energy during chemical reactions. chemical bonds have potential energy, which is converted to heat energy during chemical reactions.
Answers: 1

Chemistry, 22.06.2019 14:50
Complete the following statements to describe solids, liquids, and gases. select the correct answer from each drop-down menu. a solid a definite volume and a definite shape. a liquid a definite volume and a definite shape. a gas a definite volume and a definite shape
Answers: 1

Chemistry, 22.06.2019 21:20
One way in which the useful metal copper is produced is by dissolving the mineral azurite, which contains copper(ii) carbonate, in concentrated sulfuric acid. the sulfuric acid reacts with the copper(ii) carbonate to produce a blue solution of copper(ii) sulfate. scrap iron is then added to this solution, and pure copper metal precipitates out because of the following chemical reaction: (s) (aq) (s) (aq) suppose an industrial quality-control chemist analyzes a sample from a copper processing plant in the following way. he adds powdered iron to a copper(ii) sulfate sample from the plant until no more copper will precipitate. he then washes, dries, and weighs the precipitate, and finds that it has a mass of .
Answers: 2
You know the right answer?
Element
Molar mass (g/mol)
H
1.008
С
12.01
O
16.00
Using...
H
1.008
С
12.01
O
16.00
Using...
Questions


Health, 19.02.2021 02:30






Mathematics, 19.02.2021 02:30


Biology, 19.02.2021 02:30

Mathematics, 19.02.2021 02:30

English, 19.02.2021 02:30

Mathematics, 19.02.2021 02:30

Arts, 19.02.2021 02:30





Mathematics, 19.02.2021 02:30

Computers and Technology, 19.02.2021 02:30



