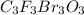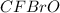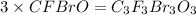Chemistry, 09.12.2019 03:31 krissymonae
What is the molecular formula of a compound if its empirical formula is cfbro and its molar mass is 381.01 g/mol?
a. cfbro
b. c2f2br2o2
c. c3f3br3o3
d. c2f3br2o3
e. c4f4br4o me

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 09:00
George is a dalmatian puppy. describe what happens to light that allows you to see george’s black and white coat.
Answers: 1

Chemistry, 22.06.2019 10:00
What is the atomic mass of an atom that has 6 protons, 6 neutrons, and 6 electrons? a) 6 b) 8 c) + 1 d) 12 e) 18
Answers: 1

Chemistry, 22.06.2019 13:00
Asubstance is a good conductor of electricity which of the following best explains a probable position of the substance in a periodic table
Answers: 3

Chemistry, 22.06.2019 17:30
A650 ml sodium bromine solution has a bromide ion concentration of 0.245 m. what is the mass (g) of sodium bromide in solution? a) 103.b)0.00155.c)16400.d) 16.4.e) 0.159
Answers: 2
You know the right answer?
What is the molecular formula of a compound if its empirical formula is cfbro and its molar mass is...
Questions


Mathematics, 26.03.2021 03:50

Biology, 26.03.2021 03:50

Mathematics, 26.03.2021 03:50

Mathematics, 26.03.2021 03:50

Business, 26.03.2021 03:50

Geography, 26.03.2021 03:50

Mathematics, 26.03.2021 03:50

Mathematics, 26.03.2021 03:50

Advanced Placement (AP), 26.03.2021 03:50

History, 26.03.2021 03:50

Business, 26.03.2021 03:50


Mathematics, 26.03.2021 03:50


History, 26.03.2021 03:50


Mathematics, 26.03.2021 03:50

Mathematics, 26.03.2021 03:50

French, 26.03.2021 03:50