
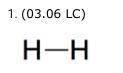
H-H Two side-by-side letter H's connected by a short, horizontal line segment. The Lewis structure for a hydrogen molecule, H2, is shown. The line segment in the structure shows that the two atoms (2 points) share one valence electron. share two valence electrons. have one unshared electron. have two unshared electrons.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 05:00
Choose all the answers that apply. ionic compounds dissolve easily in water do not dissolve in water have low melting points have high melting points conduct electricity when melted
Answers: 1

Chemistry, 22.06.2019 05:20
Temperature is _related to the average kinetic energy of a gas. inversely directly not disproportionally
Answers: 1

Chemistry, 22.06.2019 17:00
Complete each row of the table below by filling in the missing prefix or missing exponent.
Answers: 1

Chemistry, 22.06.2019 23:00
In the reaction h2co3 (aq) + 3nh3 (aq) = 2 nh4+ (aq) + co3 2-, how many electrons are transferred?
Answers: 3
You know the right answer?
H-H Two side-by-side letter H's connected by a short, horizontal line segment. The Lewis structure f...
Questions

Physics, 27.10.2020 17:10

Arts, 27.10.2020 17:10

Chemistry, 27.10.2020 17:10


Mathematics, 27.10.2020 17:10

Business, 27.10.2020 17:10



Computers and Technology, 27.10.2020 17:10

History, 27.10.2020 17:10

Mathematics, 27.10.2020 17:10

Computers and Technology, 27.10.2020 17:10


History, 27.10.2020 17:10

Chemistry, 27.10.2020 17:10




Mathematics, 27.10.2020 17:10

Biology, 27.10.2020 17:10



