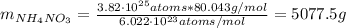Chemistry, 14.01.2021 16:40 lizbethmillanvazquez
Determine the mass of ammonium nitrate (in g) that has the same number of nitrogen atoms as 2.2 liters of liquid nitrogen (N2). Density of liquid nitrogen is 0.808 g/mL.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 17:30
You are performing an experiment in a lab to attempt a new method of producing pure elements from compounds. the only problem is that you do not know what element will form. by your previous calculations you know that you will have 6.3 moles of product. when it is complete, you weigh it and determine you have 604.4 grams. what element have you produced?
Answers: 1

Chemistry, 22.06.2019 13:30
Apush or pull that moves or changes and object when to objects touch
Answers: 2

Chemistry, 22.06.2019 16:30
4. a 20-kg child is tossed up into the air by her parent. the child is 2 meters off the ground traveling 5 m/s. circle one: ke / gpe / both show your work for finding the values of each type of energy the object has:
Answers: 1

Chemistry, 22.06.2019 18:30
When the chemicals iron sulfide (fes) and hydrochloric acid (hcl) are combined, bubbles appear from the mixture. 1. does the appearance of bubbles indicate a physical or chemical change? 2. why do the bubbles indicate this change? 3. what property is this?
Answers: 1
You know the right answer?
Determine the mass of ammonium nitrate (in g) that has the same number of nitrogen atoms as 2.2 lite...
Questions

Chemistry, 08.10.2021 22:30



English, 08.10.2021 22:30

Mathematics, 08.10.2021 22:30



Mathematics, 08.10.2021 22:30



Computers and Technology, 08.10.2021 22:30



History, 08.10.2021 22:30

Biology, 08.10.2021 22:30


Mathematics, 08.10.2021 22:30

History, 08.10.2021 22:30

Health, 08.10.2021 22:30

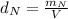
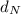 : is the density of liquid nitrogen = 0.808 g/mL
: is the density of liquid nitrogen = 0.808 g/mL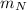 : is the mass of liquid nitrogen
: is the mass of liquid nitrogen 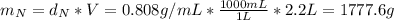
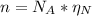
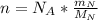
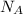 : is the Avogadro's number = 6.022x10²³ atoms/mol
: is the Avogadro's number = 6.022x10²³ atoms/mol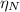 : is the number of moles of liquid nitrogen
: is the number of moles of liquid nitrogen 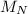 : is the molar mass of liquid nitrogen = 28.014 g/mol
: is the molar mass of liquid nitrogen = 28.014 g/mol 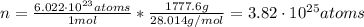
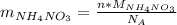
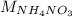 : is the molar mass of ammonium nitrate = 80.043 g/mol
: is the molar mass of ammonium nitrate = 80.043 g/mol