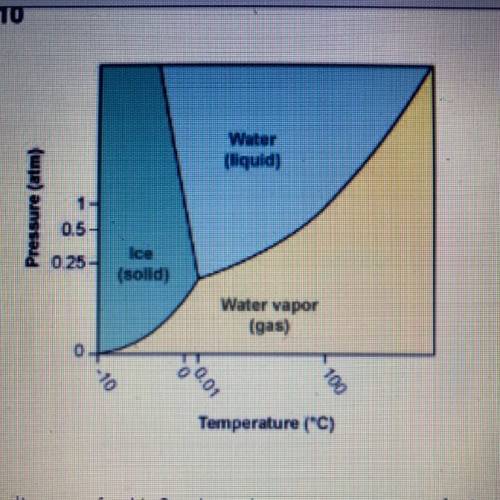
Using the phase diagram for H20, what phase is water in at 1 atm pressure
and 150°C?
A....


Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 11:00
An object becomes electrically charged when: electrons are created in it electrons from it are destroyed electrons are transferred to it protons from it are destroyed protons are created in it
Answers: 1

Chemistry, 22.06.2019 12:00
Ican determine the molar mass of an element by looking on the under the atomic mass for the element. for example the molar mass of phosphorus is 30.974 grams/mole. avogadro’s number tells me the amount of representative particles in 1 mole of any substance. this means 12.011 gram sample of carbon and a 32.0 gram sample of sulfur have the same number of atoms.
Answers: 1

Chemistry, 22.06.2019 14:30
How does a noncompetitive inhibitor reduce an enzyme’s activity?
Answers: 1

Chemistry, 23.06.2019 01:30
Will a solution form when the solvent and solute are both nonpolar? a. not likely b. never c. most likely
Answers: 1
You know the right answer?
Questions







Spanish, 18.02.2021 23:40


Mathematics, 18.02.2021 23:40

Geography, 18.02.2021 23:40


French, 18.02.2021 23:40




Mathematics, 18.02.2021 23:40

Mathematics, 18.02.2021 23:40


Mathematics, 18.02.2021 23:40

Mathematics, 18.02.2021 23:40