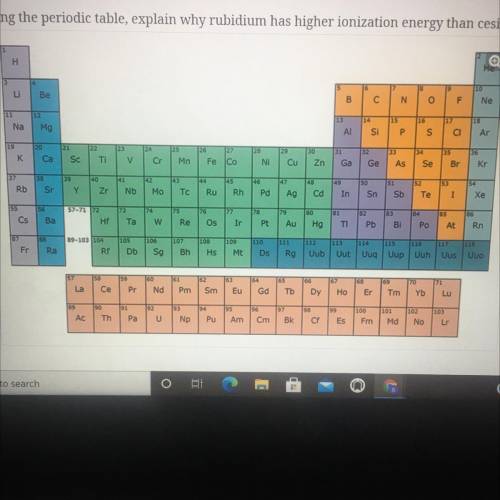
Using the periodic table, explain why rubidium has higher ionization energy than cesium.
...


Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 01:00
According to the tide table below what time of day will the highest tide occur?
Answers: 1

Chemistry, 22.06.2019 21:30
What is the effect of returning nuclear reactor cooling water back into bodies of water?
Answers: 3

Chemistry, 22.06.2019 22:30
Which of these statements best explains why space exploration should be encouraged? it prepares humans to live without oxygen. it dispel myths about objects in space. it prevents comets and asteroids from striking earth. it creates technology to absorb harmful radiations in space.
Answers: 1

Chemistry, 23.06.2019 02:20
Why dose heating increase the speed at which a solution dissolved in water
Answers: 1
You know the right answer?
Questions

Mathematics, 22.04.2021 20:10



Social Studies, 22.04.2021 20:10



Mathematics, 22.04.2021 20:10

Mathematics, 22.04.2021 20:10




Biology, 22.04.2021 20:10


Arts, 22.04.2021 20:10

English, 22.04.2021 20:10



Mathematics, 22.04.2021 20:10


History, 22.04.2021 20:10