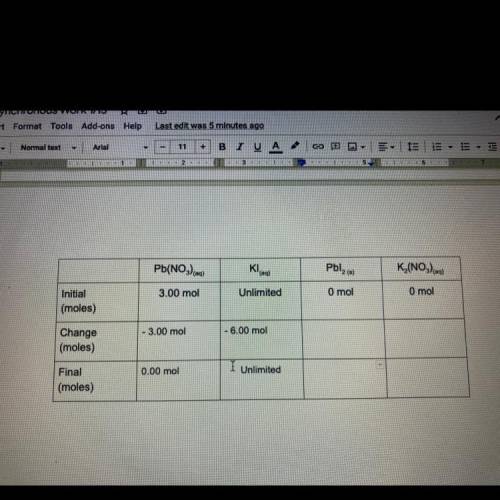
Task 4
3.00 moles of lead nitrate and excess potassium iodidq are combined in water to
form l...

Task 4
3.00 moles of lead nitrate and excess potassium iodidq are combined in water to
form lead iodide and potassium nitrate. The reaction is described by the following
chemical equation:
Pb(NO3) eq) + 2Kleg) Pblzco + K (NO)
Fill out the following table to find out how many moles of products are formed,
and how many of the reactants remain after the reaction has taken place. The boxes in
the final row are all mole to mole conversions, and can be found by using your molar
ratios and the moles of lead nitrate at the beginning of the reaction. It is your choice
how you will arrange these two numbers. If this problem becomes confusing, look back
to task 3, It's the same problem!

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 11:50
Acompound has a molecular weight of 12.124 atomic mass units and the empirical formula c3h40. what is the molecular formula of the compound?
Answers: 3

Chemistry, 22.06.2019 13:00
Asubstance is a good conductor of electricity which of the following best explains a probable position of the substance in a periodic table
Answers: 3

Chemistry, 22.06.2019 14:00
650.j is the same amount of energy as? 2720cal1550cal650.cal2.72cal
Answers: 2

Chemistry, 22.06.2019 19:00
How does a catalyst increase the speed of a reaction? a. the catalyst eliminates the activated complex stage, allowing products to form immediately. b. the catalyst lowers the energy level of the reactants, making it easier for them to react. c. the catalyst makes it easier for the activated complex to form, lowering the activation energy. d. the catalyst raises the energy level of the products, making the reaction finish sooner. reset next
Answers: 1
You know the right answer?
Questions





Mathematics, 26.03.2021 23:20

Mathematics, 26.03.2021 23:20

Mathematics, 26.03.2021 23:20

Mathematics, 26.03.2021 23:20

Mathematics, 26.03.2021 23:20

Advanced Placement (AP), 26.03.2021 23:20

Mathematics, 26.03.2021 23:20





Social Studies, 26.03.2021 23:20


Chemistry, 26.03.2021 23:20

History, 26.03.2021 23:20



