
HELP ASAPPP
An iron nail was placed in an open test tube containing some salt solution, and then left for a week for observations. The experimental setup and observations are shown below.
Which of the following best demonstrates that a chemical change has taken place?
The salt solution was discolored after a week.
The volume of the salt solution has decreased.
The mass of the salt solution, beaker and nails decreased.
The iron nail is still undissolved in the solution after a week.
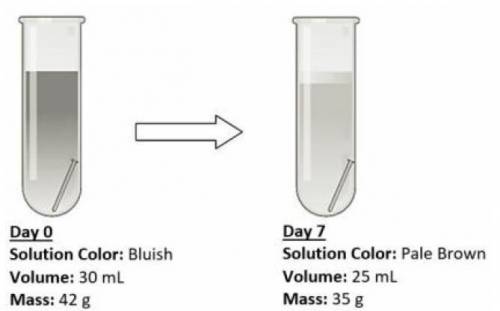

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 06:30
Melting and boiling are endothermic processes. this means that these processes absorb energy from their surroundings in order to occur. use this information and the data you collected in the phase change gizmo to describe what happens to the temperature of water when you boil it, then explain why this result occurs.
Answers: 1

Chemistry, 22.06.2019 12:20
Consider the reaction of a(g) + b(g) + c(g) => d(g) for which the following data were obtained: experiment initial [a], mol/l initial [b], mol/l initial [c], mol/l initial rate, mol/l.s 1 0.0500 0.0500 0.0100 6.25 x 10^-3 2 0.100 0.0500 0.0100 2.50 x 10^-2 3 0.100 0.100 0.0100 1.00 x 10^-1 4 0.0500 0.0500 0.0200 6.25 x 10^-3 what is the rate law for the reaction?
Answers: 3

Chemistry, 22.06.2019 13:00
The number of neutrons is equal to the atomic number minus the atomic mass. a. true b. false
Answers: 2

Chemistry, 22.06.2019 16:30
Correct relationship between molecular formula and empirical formula
Answers: 1
You know the right answer?
HELP ASAPPP
An iron nail was placed in an open test tube containing some salt solution, and then le...
Questions

Social Studies, 24.12.2020 22:50

English, 24.12.2020 22:50

English, 24.12.2020 22:50


English, 24.12.2020 22:50

Mathematics, 24.12.2020 22:50

English, 24.12.2020 22:50


Mathematics, 24.12.2020 22:50

Chemistry, 24.12.2020 22:50

Mathematics, 24.12.2020 22:50



Health, 24.12.2020 22:50


Physics, 24.12.2020 22:50


Mathematics, 24.12.2020 22:50




