Chemistry, 13.01.2021 17:10 babyjulie61
Suppose a current of is passed through an electroplating cell with an aqueous solution of agno3 in the cathode compartment for seconds. Calculate the mass of pure silver deposited on a metal object made into the cathode of the cell.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 00:00
Acurium-245 nucleus is hit with a neutron and changes as shown by the equation. complete the equation by filling in the missing parts. 52
Answers: 2

Chemistry, 22.06.2019 10:30
Which characteristics can be used to differentiate star systems? check all that apply.
Answers: 2


Chemistry, 23.06.2019 00:30
Gasoline has a density of 0.740 g/ml. if you have 328 grams of gasoline, what is the volume in milliliters?
Answers: 1
You know the right answer?
Suppose a current of is passed through an electroplating cell with an aqueous solution of agno3 in t...
Questions

Mathematics, 27.10.2020 06:50




English, 27.10.2020 06:50

Mathematics, 27.10.2020 06:50

Mathematics, 27.10.2020 06:50



Biology, 27.10.2020 06:50

Health, 27.10.2020 06:50

Mathematics, 27.10.2020 06:50

Computers and Technology, 27.10.2020 06:50




Mathematics, 27.10.2020 06:50


Mathematics, 27.10.2020 06:50

English, 27.10.2020 06:50

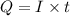
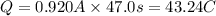
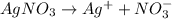
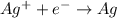
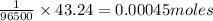 of Ag
of Ag