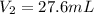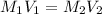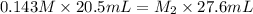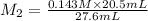Chemistry, 12.01.2021 18:00 kichensides
An aqueous solution of hydrochloric acid is standardized by titration with a 0.143 M solution of potassium hydroxide. If 20.5 mL of base are required to neutralize 27.6 mL of the acid, what is the molarity of the hydrochloric acid solution?

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 20:00
What happens to the temperature of a substance when the average kinetic energy of its particles increases?
Answers: 3

Chemistry, 22.06.2019 21:30
If you burn 46.6 g of hydrogen and produce 416 g of water, how much oxygen reacted
Answers: 3

Chemistry, 22.06.2019 21:30
Harry lives in a city, and he has a lung condition known as asthma. on certain days, harry has to stay inside because pollutants in the air make it difficult for him to breathe. which of these pollution sources are nonpoint sources that might bother harry if he goes outside? choose the two that apply.
Answers: 3

You know the right answer?
An aqueous solution of hydrochloric acid is standardized by titration with a 0.143 M solution of pot...
Questions


Mathematics, 22.06.2019 06:30


Mathematics, 22.06.2019 06:30

Mathematics, 22.06.2019 06:30

Biology, 22.06.2019 06:30




Mathematics, 22.06.2019 06:30




History, 22.06.2019 06:30

History, 22.06.2019 06:30

History, 22.06.2019 06:30

Biology, 22.06.2019 06:30

History, 22.06.2019 06:30

Biology, 22.06.2019 06:30

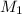 = 0.143 M,
= 0.143 M, 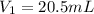
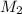 = ? ,
= ? ,