Chemistry, 12.01.2021 17:50 alexandrarosete7
The osmotic pressure exerted by a solution is equal to the molarity multiplied by the absolute temperature and the gas constant R. Suppose the osmotic pressure of a certain solution is measured to be 22 atm at an absolute temperature of 353K. Write an equation that will let you calculate the molarity c of this solution. Your equation should contain only symbols. Be sure you define each symbol other than R.

Answers: 2


Another question on Chemistry


Chemistry, 22.06.2019 12:30
Sodium sulfate dissolves as follows: na2so4(s) → 2na+(aq) + so42- (aq). how many moles of na2so4 are required to make 1.0 l of solution in which the na concentration is 0.10 m?
Answers: 2

Chemistry, 22.06.2019 22:30
What is the value of the standard enthalpy of formation of an element in its most stable form?
Answers: 3

Chemistry, 23.06.2019 00:20
How many lone pairs of electrons are on the central atom of no3- and what is the molecular shape? one, trigonal planar zero, trigonal pyramidal zero, trigonal planar one, tetrahedral one, trigonal pyramidal
Answers: 1
You know the right answer?
The osmotic pressure exerted by a solution is equal to the molarity multiplied by the absolute tempe...
Questions

History, 24.09.2020 02:01

Mathematics, 24.09.2020 02:01

English, 24.09.2020 02:01

Engineering, 24.09.2020 02:01



Mathematics, 24.09.2020 02:01



History, 24.09.2020 02:01


History, 24.09.2020 02:01


Mathematics, 24.09.2020 02:01


Mathematics, 24.09.2020 02:01

Mathematics, 24.09.2020 02:01


Mathematics, 24.09.2020 02:01

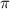 = osmotic pressure exerted by a solution
= osmotic pressure exerted by a solution