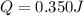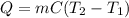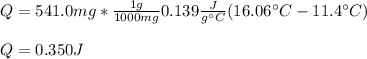Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 10:50
An atom of lithium-7 has an equal number of(1) electrons and neutrons(2) electrons and protons(3) positrons and neutrons(4) positrons and protons
Answers: 2


Chemistry, 22.06.2019 14:30
Ahypothesis must be testable and falsifiable to be considered scientific a. trueb. false
Answers: 1

Chemistry, 22.06.2019 15:00
Phosphorous acid, h3po3(aq) , is a diprotic oxyacid that is an important compound in industry and agriculture. p k a1 p k a2 1.30 6.70 calculate the ph for each of the points in the titration of 50.0 ml of 1.5 m h3po3(aq) 1.5 m h 3 po 3 ( aq ) with 1.5 m koh(aq). 1.5 m koh ( aq ) .
Answers: 1
You know the right answer?
Calculate the energy required to heat 541.0mg of mercury from 11.4°C to 16.6°C. Assume the specifi...
Questions

Biology, 31.08.2019 12:30

English, 31.08.2019 12:30


Biology, 31.08.2019 12:30


Biology, 31.08.2019 12:30







Mathematics, 31.08.2019 12:30

Mathematics, 31.08.2019 12:30

Mathematics, 31.08.2019 12:30

Mathematics, 31.08.2019 12:30

Physics, 31.08.2019 12:30