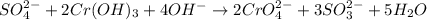Answers: 3


Another question on Chemistry



Chemistry, 22.06.2019 02:00
How many moles of magnesium is 3.01 x10^22 atoms of magnesium?
Answers: 1

Chemistry, 22.06.2019 09:00
At 300 mm hg, a gas has a volume of 380 l, what is the volume at standard pressure
Answers: 1
You know the right answer?
SO42- + 2Cr(OH)3 + 4OH- → + 2CrO42- + 3SO32- + 5H2O
In the above redox reaction. Use oxidation nu...
Questions


English, 29.05.2020 22:00

Mathematics, 29.05.2020 22:00

Biology, 29.05.2020 22:00

History, 29.05.2020 22:00


Mathematics, 29.05.2020 22:00

Mathematics, 29.05.2020 22:00


History, 29.05.2020 22:00

Mathematics, 29.05.2020 22:00


Mathematics, 29.05.2020 22:01

Mathematics, 29.05.2020 22:01


Computers and Technology, 29.05.2020 22:01


Mathematics, 29.05.2020 22:01

Mathematics, 29.05.2020 22:01