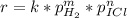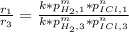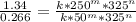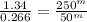Chemistry, 12.01.2021 17:40 ineedhelpasap12
Given the following data, determine the order of reaction with respect to H2.
H2(g)+ 2ICI → I2(g)+ 2HCL(g)
Experiment [H2](torr) ICI[torr] Rate(M/s)
1 250 325 1.34
2 250 81 0.331
3 50 325 0.266

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 21:40
During trial 2, what allowed you to determine that aluminum was the limiting reactant? check all that apply. all of the copper dissolved. all of the aluminum dissolved. the solution turned clear. the number of grams of copper(ii) chloride used in the reaction was greater than the number of grams of aluminum. the molar ratio of copper(ii) chloride to aluminum was greater than 3: 2, the equation’s molar ratio.
Answers: 2

Chemistry, 22.06.2019 04:30
Acamcorder has a power rating of 17 watts. if the output voltage from its battery is 7 volts, what current does it use?units:
Answers: 1


Chemistry, 22.06.2019 20:30
Which states of matter have particles that move independently of one another with very little attraction?
Answers: 1
You know the right answer?
Given the following data, determine the order of reaction with respect to H2.
H2(g)+ 2ICI → I2(g)...
Questions


Mathematics, 03.02.2022 14:00

SAT, 03.02.2022 14:00

SAT, 03.02.2022 14:00

Spanish, 03.02.2022 14:00




Mathematics, 03.02.2022 14:00


Mathematics, 03.02.2022 14:00


Computers and Technology, 03.02.2022 14:00


English, 03.02.2022 14:00

SAT, 03.02.2022 14:00

Mathematics, 03.02.2022 14:00


Mathematics, 03.02.2022 14:00

Mathematics, 03.02.2022 14:00