Calculate the morality for the following
10.5 kg of Na2 SO4. 10H2O in 18.60 L...

Chemistry, 12.01.2021 16:50 zafarm2oxgpmx
Calculate the morality for the following
10.5 kg of Na2 SO4. 10H2O in 18.60 L

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 23:50
2points what is the job of a scientist? a. to answer ethical questions. b. to write laws based on his or her knowledge. c. to ask and answer scientific questions. d. to ignore facts that do not support his or her theory.
Answers: 1


Chemistry, 22.06.2019 11:00
Which statement correctly identifies the scientific question and describes why the question is scientific? question 1 refers to the supernatural.question 2 reflects a moral or social value.question 3 refers to something that can be measured.question 4 reflects a question that can’t be observed.
Answers: 1

Chemistry, 23.06.2019 01:30
What is produced from neutralization of an acid and a base? a. hydronium ions b. citric acid c. salt and water
Answers: 1
You know the right answer?
Questions

Mathematics, 24.03.2021 21:10


Biology, 24.03.2021 21:10



Physics, 24.03.2021 21:10


Health, 24.03.2021 21:10

Mathematics, 24.03.2021 21:10






Mathematics, 24.03.2021 21:10





Mathematics, 24.03.2021 21:10

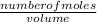
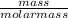
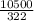 = 32.6mole
= 32.6mole 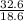 = 1.75M
= 1.75M

