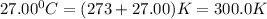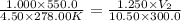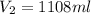Chemistry, 12.01.2021 07:40 nanakwameyeb
5. 4.50 moles of a certain gas occupies a volume of 550.0 mL at 5.000ºC and 1.000 atm. What would the volume be if 10.50 moles are present at 27.00°C and 1.250 atm? HELPPP

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 03:30
If a solution is considered basic, then a) the hydroxide ion and hydronium ion concentrations are equal. b) the hydroxide ion concentration is less than the hydronium ion concentration. c) the hydronium ion concentration is greater than the hydroxide ion concentration. d) the hydroxide ion concentration is greater than the hydronium ion concentration.
Answers: 1


Chemistry, 22.06.2019 12:00
Consider the following reaction at equilibrium. 2co2 (g) 2co (g) + o2 (g) h° = -514 kj le châtelier's principle predicts that the equilibrium partial pressure of co (g) can be maximized by carrying out the reaction a. at high temperature and high pressure b. at high temperature and low pressure c. at low temperature and low pressure d. at low temperature and high pressure e. in the presence of solid carbon
Answers: 2

Chemistry, 23.06.2019 05:50
Aseismic wave is energy released as the result of rock movement along a fault. t or f ?
Answers: 1
You know the right answer?
5. 4.50 moles of a certain gas occupies a volume of 550.0 mL at 5.000ºC and 1.000 atm. What would th...
Questions


Mathematics, 20.07.2020 09:01

Mathematics, 20.07.2020 09:01

Mathematics, 20.07.2020 09:01

Mathematics, 20.07.2020 09:01





Physics, 20.07.2020 09:01






History, 20.07.2020 09:01

Mathematics, 20.07.2020 09:01

History, 20.07.2020 09:01

Social Studies, 20.07.2020 09:01

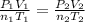
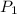 = initial pressure = 1.000 atm
= initial pressure = 1.000 atm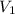 = initial volume = 550.0 ml
= initial volume = 550.0 ml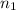 = initial moles = 4.50
= initial moles = 4.50 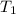 = initial temperature =
= initial temperature = 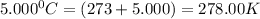
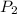 = final pressure = 1.250 atm
= final pressure = 1.250 atm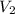 = final volume = ?
= final volume = ?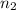 = final moles = 10.50
= final moles = 10.50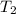 = final temperature =
= final temperature =