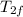Chemistry, 12.01.2021 04:30 hannah2718
Fuel
1
fuel
1
phase gas
phase: liquid
energy
transferred
out
fuel
2
fuel
2
phase, gas
phase: gps
engine OFF
engine ON
A certain type of ship has two tanks in its engine. Each tank contains a different
type of fuel. When the engine turns on, the same amount of energy is transferred
out of both fuels as shown in the diagram below. Why did fuel 1 change phase,
but fuel 2 stayed the same? Explain what happened to the molecules of both
fuels,

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 14:30
Ahypothesis must be testable and falsifiable to be considered scientific a. trueb. false
Answers: 1

Chemistry, 23.06.2019 00:30
What is bromine+calcium iodide--> calcium bromide +iodine balanced
Answers: 1

Chemistry, 23.06.2019 02:30
What type of energy conversion occurs when you place your feet near the fire place and they become warm
Answers: 1

Chemistry, 23.06.2019 04:20
The reaction below shows a system in equilibrium. how would a decrease in temperature affect this reaction? a. the rate of formation of the gases would increase. b. the equilibrium of the reaction would shift to the left. c. the equilibrium would shift to cause the gases to sublime into solids. d. the chemicals on the left would quickly form the chemical on the right.
Answers: 1
You know the right answer?
Fuel
1
fuel
1
phase gas
phase: liquid
energy
transferred
...
fuel
1
phase gas
phase: liquid
energy
transferred
...
Questions


Geography, 27.02.2020 21:59

English, 27.02.2020 21:59


English, 27.02.2020 21:59



English, 27.02.2020 21:59









English, 27.02.2020 21:59

Chemistry, 27.02.2020 21:59


Geography, 27.02.2020 21:59

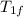 will be lower than the final temperature of fuel 2,
will be lower than the final temperature of fuel 2,