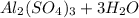Chemistry, 11.01.2021 22:30 JessTaylr04
For the following reaction, 3.04 grams of sulfuric acid are mixed with excess aluminum oxide. The reaction yields 2.53 grams of aluminum sulfate. aluminum oxide (s) sulfuric acid (aq) aluminum sulfate (aq) water (l) What is the theoretical yield of aluminum sulfate

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 10:10
When electrolyzing copper (ll) chloride, what reaction takes place at the anode? what reaction takes place at the cathode?
Answers: 1


Chemistry, 22.06.2019 12:30
If 22.5 liters of oxygen reacted with excess of hydrogen, how many liters of water vapor could be produced?
Answers: 3

Chemistry, 22.06.2019 14:30
Select the word from the list that best fits the definition the nuclear family into which a person is born or adopted.
Answers: 2
You know the right answer?
For the following reaction, 3.04 grams of sulfuric acid are mixed with excess aluminum oxide. The re...
Questions



Business, 02.11.2020 20:40




Law, 02.11.2020 20:40

English, 02.11.2020 20:40

History, 02.11.2020 20:40



Social Studies, 02.11.2020 20:40

Mathematics, 02.11.2020 20:40

English, 02.11.2020 20:40


English, 02.11.2020 20:40


Mathematics, 02.11.2020 20:40

Spanish, 02.11.2020 20:40

 →
→