
Chemistry, 15.01.2020 15:31 aidanfbussiness
1. in the reaction c5h8 + o2 --> 8h2o + 5co2, what coefficient should be placed in front of o2 to balance the reaction?
a. 5
b. 8
c. 9
d. no coefficient is needed
2. how does the law of conservation of mass apply to this reaction: c2h4 + o2 --> 2h2o + 2co2?
a. each element needs to be balanced.
b. the equation needs to be balanced. there are fewer oxygen atons in the equation than hydrogen or carbon.
c. only the oxygen needs to be balanced. there are equal numbers of hydrogen and carbon.
d. the law of conservation of mass has already been applied. there is an equal number of each element on both sides of the equation
3. how does the law of conservation of mass apply to this reaction: mg + hcl --> h2 + mgcl2?
a. the law of conservation of mass has already been applied. there is an equal number of each element on both sides of the equation.
b. only the hydrogen needs to be balanced. there are equal amount of magnesium and chlorine.
c. hydrogen and chlorine need to be balanced. there is an equal amount of magnesium on each side.
d. the equation needs to be balanced. there are a few hydrogen atoms in the equation than magnesium or chlorine.
4. which of these is an example of a solution?
a. oil and vinegar in a salad dressing jar
b. a glass of water with ice in it
c. sugar stirred in tea
d. sand stirred in water

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 12:00
What is the percentage of hydrogen in nitrogen trihydride
Answers: 1

Chemistry, 22.06.2019 23:00
Consider the reaction: 2al(s) + fe2o3(s) → al2o3(s) + 2fe(s) the δhf for fe2o3(s) = -824.3 kj/mole. the δhf for al2o3(s) = -1675.7 kj/mole. finish the equation. δhrxn = [(1)( kj/mole) + (2)( kj/mole)] - [(1)( kj/mole) + (2) ( kj/mole)]
Answers: 1

Chemistry, 23.06.2019 02:40
Calculate the standard enthalpy of formation of liquid methanol, ch3oh(l), using the following information: c(graphite) + o2 latex: \longrightarrow ⟶ co2(g) latex: \delta δ h° = –393.5 kj/mol h2(g) + o2 latex: \longrightarrow ⟶ h2o(l) latex: \delta δ h° = –285.8 kj/mol ch3oh(l) + o2(g) latex: \longrightarrow ⟶ co2(g) + 2h2o(l) latex: \delta δ h° = –726.4 kj/mol
Answers: 3

Chemistry, 23.06.2019 03:30
Name 3 types of energy you see being used as you look around a classroom
Answers: 1
You know the right answer?
1. in the reaction c5h8 + o2 --> 8h2o + 5co2, what coefficient should be placed in front of o2...
Questions

Mathematics, 22.07.2019 02:00




Social Studies, 22.07.2019 02:00




History, 22.07.2019 02:00

Social Studies, 22.07.2019 02:00




Mathematics, 22.07.2019 02:00


Mathematics, 22.07.2019 02:00


Social Studies, 22.07.2019 02:00

Health, 22.07.2019 02:00

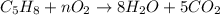
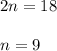
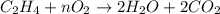
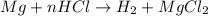
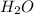 So, this is not considered as a solution.
So, this is not considered as a solution.

