
Chemistry, 11.01.2021 04:10 makennahudson94
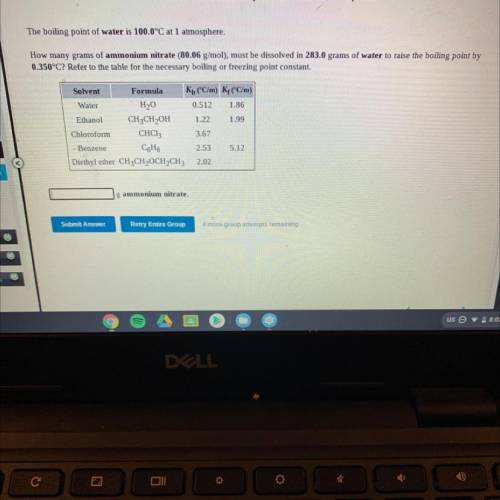
The boiling point of water is 100.0°C at 1 atmosphere.
How many grams of ammonium nitrate (80.06 g/mol), must be dissolved in 283.0 grams of water to raise the boiling point by
0.350°C? Refer to the table for the necessary boiling or freezing point constant.

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 21:30
If i make a solution by adding 83grams of sodium hydroxide to 750ml i’d water what is the molarity of sodium hydroxide
Answers: 1

Chemistry, 22.06.2019 04:00
Write the balanced equation for a reaction between aqueous nitric acid (hno3) and solid lithium metal (this is a single replacement reaction)
Answers: 1

Chemistry, 22.06.2019 05:00
When you mate two plants together the terms is called? answer it fast as possible plz! i have a test tomorrow!
Answers: 1

Chemistry, 22.06.2019 05:50
According to coulomb's law, how would the electrical force between particles change if the product of their electrical charge increased?
Answers: 1
You know the right answer?
The boiling point of water is 100.0°C at 1 atmosphere.
How many grams of ammonium nitrate (80.06 g/...
Questions


Biology, 08.01.2021 04:00

German, 08.01.2021 04:10


Mathematics, 08.01.2021 04:10

Mathematics, 08.01.2021 04:10

Mathematics, 08.01.2021 04:10

Mathematics, 08.01.2021 04:10


Biology, 08.01.2021 04:10

Mathematics, 08.01.2021 04:10

Mathematics, 08.01.2021 04:10

Chemistry, 08.01.2021 04:10

History, 08.01.2021 04:10


History, 08.01.2021 04:10

Biology, 08.01.2021 04:10


Mathematics, 08.01.2021 04:10

Social Studies, 08.01.2021 04:10



