
Chemistry, 11.01.2021 03:40 qveenjordan6456
Suppose 275 g of NO3- flows into a swamp each day. What volume of CO2 would be produced each day at 17.0°C and 1.00 atm? I attached the image of the equation for reference


Answers: 2


Another question on Chemistry


Chemistry, 22.06.2019 23:30
How many grams of ammonia would be produced by the decomposition of 16.93 mlof hydrazine? (the density of hydrazine is 1.021g/ml)
Answers: 3

Chemistry, 23.06.2019 02:00
Which best describes the present-day universe? opaque, expanding very slowly, stars produce heavy elements transparent, expanding at an accelerated rate, stars produce heavy elements opaque, expanding at an accelerated rate, stars produce only hydrogen and helium transparent, expanding very slowly, stars produce only hydrogen and helium
Answers: 1

Chemistry, 23.06.2019 05:00
What is dhmo? hint: you find it everywhere something is wet..
Answers: 1
You know the right answer?
Suppose 275 g of NO3- flows into a swamp each day. What volume of CO2 would be produced each day at...
Questions





Social Studies, 01.01.2021 21:30


Mathematics, 01.01.2021 21:30





English, 01.01.2021 21:40



Mathematics, 01.01.2021 21:40

Mathematics, 01.01.2021 21:40



Computers and Technology, 01.01.2021 21:40

Mathematics, 01.01.2021 21:40

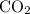 produced has been 264.28 L.
produced has been 264.28 L.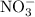 has been:
has been: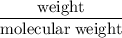
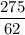
 Volume = moles
Volume = moles 

