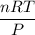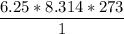Chemistry, 10.01.2021 18:30 kaylaamberd
En un matraz, disponemos de 100 g de gas oxígeno que se encuentran a 1 at de presión y 273 K de temperatura. Calcular : a) el número de moles de gas oxígeno contenidos en el matraz ; b) el número de moléculas de oxígeno ; c) el número de átomos de oxígeno ; d) el volumen ocupado por el oxígeno. Masa atómica del oxígeno = 16.

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 14:10
The rock in a lead ore deposit contains 89 % pbs by mass. how many kilograms of the rock must be processed to obtain 1.5 kg of pb?
Answers: 1

Chemistry, 22.06.2019 20:00
I’m an electrically neutral atomic any element, there are equal numbers of
Answers: 2

Chemistry, 22.06.2019 21:00
As we move from left to right across the periodic table, what is the general trend? a) atomic radii increase. b) electronegavitiy decreases. c) nuclear shielding increases. d) metallic character decreases.
Answers: 1

Chemistry, 23.06.2019 02:30
Asubstance is held in an open container. its particles move past one another at random speeds but do not leave the container. heat is removed from the system, and the particles slow down. when enough heat is removed, the particles no longer have enough speed to overcome the weak attractive forces between them. when this happens, the substance enters its solid state. the process described above is known as .
Answers: 3
You know the right answer?
En un matraz, disponemos de 100 g de gas oxígeno que se encuentran a 1 at de presión y 273 K de temp...
Questions


Mathematics, 21.05.2021 01:00


History, 21.05.2021 01:00

Mathematics, 21.05.2021 01:00




Mathematics, 21.05.2021 01:00






Mathematics, 21.05.2021 01:00

Mathematics, 21.05.2021 01:00

Mathematics, 21.05.2021 01:00