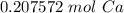How many moles of Ca are in 1.25x1023 atoms?
We used two conversion factors in this unit, they are provided below. It is your responsibility to know which conversion factor to use!
Avogadro’s number: 6.02x1023 atoms = 1 mole
Molar mass of calcium: 40.078 g Ca = 1 mol Ca
A. 3.12x10^21 mol
B. 0.21 mol
C. 3.12 mol
D. 0.21x10^21 mol

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 02:30
Margaret wants to make an orange flavored drink by stirring powdered drink mix into a glass of water. she doesn't like drinks that have small clumps of powdered solid in them, so she wants the drink to be a perfect solution. what factors should margaret not consider when deciding how much powder to add to her glass of water?
Answers: 3


Chemistry, 23.06.2019 05:00
Question 5 match each term to its description. match term definition excess reactant a) reactant that can produce a lesser amount of the product limiting reactant b) reactant that can produce more of the product theoretical yield c) amount of product predicted to be produced by the given reactants
Answers: 2

Chemistry, 23.06.2019 10:30
When a chemist collects hydrogen gas over water, she ends up with a mixture of hydrogen and water vapor in her collecting bottle if the pressure in the collecting bottle is 97.1 kilopascals and the vapor pressure of the water is 3 2 kilopascals, what is the partial pressure of the hydrogen?
Answers: 1
You know the right answer?
How many moles of Ca are in 1.25x1023 atoms?
We used two conversion factors in this unit, they are...
Questions


Mathematics, 10.11.2020 01:00

Mathematics, 10.11.2020 01:00



French, 10.11.2020 01:00


English, 10.11.2020 01:00

Mathematics, 10.11.2020 01:00

Mathematics, 10.11.2020 01:00

Social Studies, 10.11.2020 01:00

Mathematics, 10.11.2020 01:00



Mathematics, 10.11.2020 01:00

Mathematics, 10.11.2020 01:00



World Languages, 10.11.2020 01:00

Business, 10.11.2020 01:00

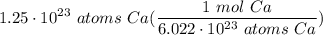 Multiply:
Multiply: