Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 14:00
Acar tire has a pressure of 2.38 atm at 15.2 c. if the pressure inside reached 4.08 atm, the tire will explode. how hot would the tire have to get for this to happen? report the temperature in degrees celsius.
Answers: 2

Chemistry, 22.06.2019 23:00
What is the formula of the ionic compound composed of calcium cations and chloride anions
Answers: 1


Chemistry, 23.06.2019 00:20
Steam reforming of methane ( ch4) produces "synthesis gas," a mixture of carbon monoxide gas and hydrogen gas, which is the starting point for many important industrial chemical syntheses. an industrial chemist studying this reaction fills a 1.5 l flask with 3.5 atm of methane gas and 1.3 atm of water vapor at 43.0°c. he then raises the temperature, and when the mixture has come to equilibrium measures the partial pressure of carbon monoxide gas to be 1 .0 atm. calculate the pressure equilibrium constant for the steam reforming of methane at the final temperature of the mixture. round your answer to 2 significant digits.
Answers: 1
You know the right answer?
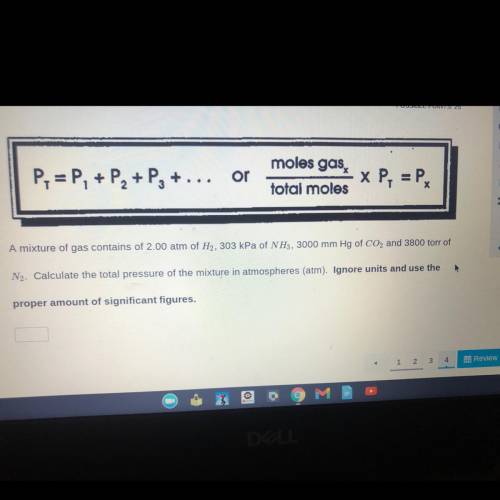
will give brainliest!! please help ! .. Calculate the total pressure of the mixture in atmospheres (...
Questions

Spanish, 23.10.2020 16:40


Mathematics, 23.10.2020 16:40

Mathematics, 23.10.2020 16:40

Mathematics, 23.10.2020 16:40


Social Studies, 23.10.2020 16:40

English, 23.10.2020 16:40

Advanced Placement (AP), 23.10.2020 16:40

Mathematics, 23.10.2020 16:40

Biology, 23.10.2020 16:40


Arts, 23.10.2020 16:40

Mathematics, 23.10.2020 16:40


History, 23.10.2020 16:40

Mathematics, 23.10.2020 16:40

Mathematics, 23.10.2020 16:40

Health, 23.10.2020 16:40