bMercury-198

Chemistry, 08.01.2021 21:00 trishinada63
Identify the missing particle in the following nuclear equation.
aPlatinum-199
bMercury-198
cMercury-199
dPlatinum-198
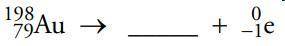

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 21:30
The reaction q+r2=r2q is found to be first order in r2 and
Answers: 1

Chemistry, 21.06.2019 22:30
Which statement best describes the oxidation numbers of the atoms found in magnesium chloride? a. magnesium has a 2- oxidation number and chlorine has a 1+ oxidation number. b. magnesium has a 2- oxidation number and chlorine has a 2+ oxidation number. c. magnesium has a 2+ oxidation number and chlorine has a 1- oxidation number. d. magnesium has a 1+ oxidation number and chlorine has a 1- oxidation number.
Answers: 2


Chemistry, 22.06.2019 15:20
Identify arrows pointing to bonding electrons. done h-0-0-h ) intro
Answers: 3
You know the right answer?
Identify the missing particle in the following nuclear equation.
aPlatinum-199
bMercury-198
bMercury-198
Questions



Spanish, 07.05.2021 20:50


Mathematics, 07.05.2021 20:50

Mathematics, 07.05.2021 20:50



English, 07.05.2021 20:50

Mathematics, 07.05.2021 20:50


Biology, 07.05.2021 20:50



Mathematics, 07.05.2021 20:50

Mathematics, 07.05.2021 20:50

Mathematics, 07.05.2021 20:50

Mathematics, 07.05.2021 20:50




