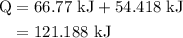Chemistry, 20.11.2019 19:31 Masielovebug
Heat is added to a 200.-gram sample of h2o(s) to melt the sample at 0°c. then the resulting h2o ( ) is heated to a final temperature of 65°c. determine the total amount of heat required to completely melt the sample. heat is added to a 200.-gram sample of h2o(s) to melt the sample at 0°c. then the resulting h2o ( ) is heated to a final temperature of 65°c. determine the total amount of heat required to completely melt the sample.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 02:30
What is the relation between concentration of reactants and the rate of chemical reaction?
Answers: 1

Chemistry, 22.06.2019 14:30
Amixture that has two or more substances that are spread out evenly is called a. compound b. heterogeneous c. substance d. homogeneous
Answers: 1

Chemistry, 22.06.2019 17:40
How much heat is added if 0.814g of water increase in temperature by 0.351 degree c?
Answers: 3

Chemistry, 22.06.2019 22:00
Choose all the answers that apply. fluorine (f) has an atomic number of 9 and an atomic weight of 18.99. fluorine has a. 9 protons b. 10 neutrons c. 18 electrons d. an atomic mass of 19 e. at least one isotope
Answers: 1
You know the right answer?
Heat is added to a 200.-gram sample of h2o(s) to melt the sample at 0°c. then the resulting h2o ( )...
Questions


English, 05.07.2019 15:00





History, 05.07.2019 15:00


English, 05.07.2019 15:00

Mathematics, 05.07.2019 15:00




Spanish, 05.07.2019 15:00

Mathematics, 05.07.2019 15:00


Mathematics, 05.07.2019 15:00


Mathematics, 05.07.2019 15:00

Mathematics, 05.07.2019 15:00

 of energy is required to melt the sample completely.
of energy is required to melt the sample completely.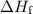 .
.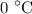 is converted to water at
is converted to water at 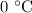 .
.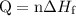 ...... (1)
...... (1) 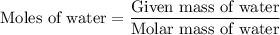 ...... (2)
...... (2) 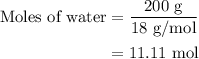
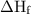 in equation (1).
in equation (1).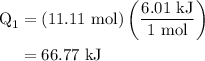
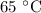 .
.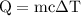 …… (3)
…… (3) 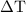 is the temperature change.
is the temperature change.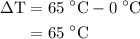
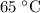 for
for 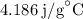 for c in equation (3).
for c in equation (3).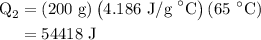
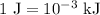
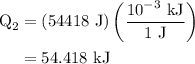
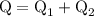 ...... (4)
...... (4) 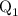 ,and
,and 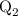 are the values of energies calculated in first and second step respectively.
are the values of energies calculated in first and second step respectively.