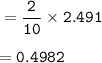Chemistry, 08.01.2021 06:10 ErikHabdlowich
How many moles of butane (C4H10(g)) were combusted in the presence of excess oxygen gas if 55.8 L of water vapour at STP is collected?

Answers: 3


Another question on Chemistry


Chemistry, 22.06.2019 18:00
An object displaces 652 ml of water. the volume of the object is: 0.652 cm³ 6.52 cm³ 65.2 cm³ 652 cm³
Answers: 2

Chemistry, 22.06.2019 19:00
How many liters of ethylene glycol antifreeze (c2h6o2), with a density of 1.100 g/l, would you add to your car radiator containing 15.0 kg of water if you needed to protect your engine to - 21.5°c? for water, kf = 1.86°c m -1.
Answers: 1

Chemistry, 22.06.2019 21:30
Liquid ammonia is produced at high temperatures and under great pressure in a tank by passing a mixture of nitrogen gas and hydrogen gas over an iron catalyst. the reaction is represented by the following equation. n2(g) + 3h2(g) → 2nh3(g) changing all but one experimental condition will affect the amount of ammonia produced. that condition is a) increasing the concentration of both reactants b) changing the temperature within the tank c) decreasing the pressure within the tank. d) increasing only the amount of nitrogen present.
Answers: 1
You know the right answer?
How many moles of butane (C4H10(g)) were combusted in the presence of excess
oxygen gas if 55.8 L o...
Questions

Mathematics, 03.06.2021 18:00


History, 03.06.2021 18:00


Mathematics, 03.06.2021 18:00

Mathematics, 03.06.2021 18:00



Mathematics, 03.06.2021 18:00




Mathematics, 03.06.2021 18:00

Mathematics, 03.06.2021 18:00

Mathematics, 03.06.2021 18:00

Mathematics, 03.06.2021 18:00


Chemistry, 03.06.2021 18:00