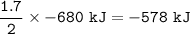Chemistry, 08.01.2021 01:00 annapittbull12
48 g of Aluminum will produce how much heat (in kJ) for this reaction?
Use the following reaction:
2 Al + 3 CuSO4 → 3 Cu + Al2(SO4)3 ∆H = -680 kJ
Round your answer to a positive whole number and do not write the unit.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 10:30
When the speed of the bottle is 2 m/s, the average maximum height of the beanbag is m.
Answers: 2

Chemistry, 22.06.2019 14:00
The content of manganese (mn) in steel was determined spectrophotometrically and with the use of the standard addition method. an unknown sample of mn from a digested steel sample gave an absorbance of 0.185 when analyzed spectrophotometrically. when 5.00 ml of solution containing 95.5 ppm mn was added to 50.0 ml of the unknown steel solution (digested sample), the absorbance was 0.248. calculate the concentration, in parts-per-million (ppm), of mn in the digested steel sample solution.
Answers: 3

Chemistry, 22.06.2019 16:50
Answer asap need by wednesday morning calculate the ph of 0.036m naoh best answer will be brainliest
Answers: 3

Chemistry, 22.06.2019 19:30
How might this scientific phenomena be explained? a paper clip floats on water.
Answers: 1
You know the right answer?
48 g of Aluminum will produce how much heat (in kJ) for this reaction?
Use the following reaction:<...
Questions


Mathematics, 24.04.2020 21:35







Mathematics, 24.04.2020 21:36

Mathematics, 24.04.2020 21:36


English, 24.04.2020 21:36

Spanish, 24.04.2020 21:36


History, 24.04.2020 21:36


Mathematics, 24.04.2020 21:36

Mathematics, 24.04.2020 21:36


English, 24.04.2020 21:36