
Chemistry, 07.01.2021 21:30 michellealvarez985
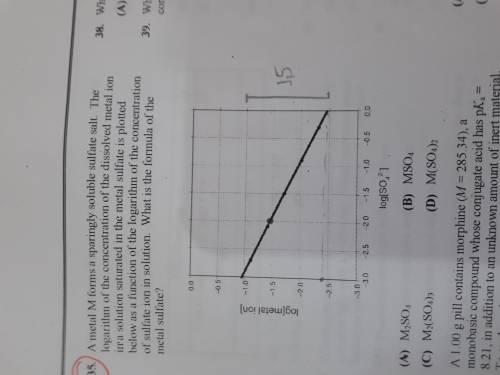
A metal M forms a sparingly soluble sulfate salt. The logarithm of the concentration of the dissolved metal ion in a solution saturated in the metal sulfate is plotted bellow as a function of the logarithm of the concentration of sulfate ion in solution. What is the formula of the metal sulfate?
a)M2SO4
b)MSO4
c)M2(SO4)3
d) M(SO4)2

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 17:00
Complete each row of the table below by filling in the missing prefix or missing exponent.
Answers: 1

Chemistry, 23.06.2019 07:00
Scuba divers use tanks of compressed air to them breathe. gases can be compressed because?
Answers: 1


Chemistry, 23.06.2019 12:30
How does a nuclear reactor produce electricity? a. high-energy gamma rays are converted by a generator into electricity. b. the heat from the reaction turns water to steam, which runs a generator. c. the reaction produces a stream of electrons that flow through wires and into batteries. d. the heat released from the reaction is used to burn coal or gas to produce electricity. e. control rods absorb the neutrons emitted and release a stream of electrons as electricity.
Answers: 1
You know the right answer?
A metal M forms a sparingly soluble sulfate salt. The logarithm of the concentration of the dissolve...
Questions


Computers and Technology, 05.02.2020 01:51

English, 05.02.2020 01:51

Mathematics, 05.02.2020 01:52



Chemistry, 05.02.2020 01:52



Chemistry, 05.02.2020 01:52


Chemistry, 05.02.2020 01:52


Computers and Technology, 05.02.2020 01:52



Mathematics, 05.02.2020 01:52





