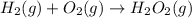Chemistry, 07.01.2021 20:10 cmarton30140
Write a balanced chemical equation depicting the formation of one mole of H2O2(g) from its elements in their standard states.

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 15:40
Achemistry student weighs out of phosphoric acid , a triprotic acid, into a volumetric flask and dilutes to the mark with distilled water. he plans to titrate the acid with solution. calculate the volume of solution the student will need to add to reach the final equivalence point. round your answer to significant digits.
Answers: 3

Chemistry, 22.06.2019 04:30
How many moles of air are there in a human lung with a volume of 2.4 l at stp? explain your answer
Answers: 1

Chemistry, 22.06.2019 10:00
Water's surface tension and heat storage capacity are accounted for by its a) orbitals b) weight c) hydrogen bonds d) mass e) size
Answers: 2

Chemistry, 22.06.2019 12:00
What term is applied to a scientist who studies ancient life, including animal and plant fossils a. anthropologist b. dendroclimatologist c. geophysicist d. paleontologist
Answers: 2
You know the right answer?
Write a balanced chemical equation depicting the formation of one mole of H2O2(g) from its elements...
Questions

Chemistry, 01.05.2021 20:10


Advanced Placement (AP), 01.05.2021 20:10






Mathematics, 01.05.2021 20:10

Mathematics, 01.05.2021 20:10

Business, 01.05.2021 20:10



English, 01.05.2021 20:10






Mathematics, 01.05.2021 20:10