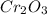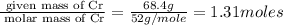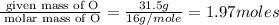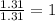Chemistry, 07.01.2021 17:30 elizabethatkins1922
Measurements show that unknown compound X has the following composition: element mass % chromium 68.4% oxygen 31.5% Write the empirical chemical formula of X.

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 20:30
Five students had to answer the question how are elements arranged in a periodic tabledamon: i think the elements are arranged by increasing massflo: i think the elements are arranged according to their properties sienna: i think the elements are arranged by when their discovers kyle: i think the elements are arranged according to how common they areglenda: i don't agree with any of themwho is right
Answers: 1

Chemistry, 22.06.2019 07:30
Free answer. the treaty of versailles ended world war i, but some of the terms of the treaty contributed to the beginning of world war ii. which was one of the terms of the treaty? the answer would be "germany was forces to pay reparations to the allied countries.". i hope this .
Answers: 1

Chemistry, 22.06.2019 09:50
Although respiratory organs vary across different organisms, they all contain respiratory surfaces that have a large surface area and are extremely thin. explain why having an extremely thin respiratory surface with a large surface area is advantageous for the process of gas exchange
Answers: 1

Chemistry, 22.06.2019 14:00
Calculate the frequency of a wave in a spring toy. the wave has a speed of 1.1 meters per second and a wavelength of 0.1 meters. *
Answers: 2
You know the right answer?
Measurements show that unknown compound X has the following composition: element mass % chromium 68....
Questions

Mathematics, 14.04.2020 22:56

Health, 14.04.2020 22:56

Arts, 14.04.2020 22:56

History, 14.04.2020 22:56

Mathematics, 14.04.2020 22:56


History, 14.04.2020 22:56

English, 14.04.2020 22:56

Mathematics, 14.04.2020 22:56

Mathematics, 14.04.2020 22:56

History, 14.04.2020 22:56


Mathematics, 14.04.2020 22:56


English, 14.04.2020 22:56


Mathematics, 14.04.2020 22:56


Mathematics, 14.04.2020 22:56