
Chemistry, 07.01.2021 05:20 Justinoreilly71
A 4.0g Glass was heated from 5°C to 45°C after absorbing 32 J of heat. What is the specific heat of the glass? 

Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 00:40
During which time interval does the object travel approximately 10 meters
Answers: 3

Chemistry, 22.06.2019 15:30
Two metal blocks that have slightly different temperatures are placed next to one another. after five minutes, they both have lower but equal temperatures. according to the law of conservation of energy, what most likelyhappened? energy was created inside the blocks.energy was destroyed inside the blocks.energy was absorbed into the blocks from outside the system.energy was transferred from the warmer block to the cooler block.
Answers: 2

Chemistry, 22.06.2019 22:00
How many moles of no2 will form when 3.3 moles of cu are reacted with excess hno3?
Answers: 3
You know the right answer?
A 4.0g Glass was heated from 5°C to 45°C after absorbing 32 J of heat. What is the specific heat of...
Questions









Mathematics, 05.05.2020 18:18


History, 05.05.2020 18:18









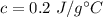
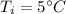
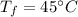
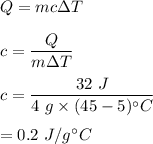
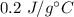 .
.

