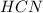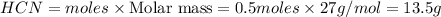Methane (CH4), ammonia (NH3), and oxygen (O2) can react to form hydrogen cyanide (HCN) and water according to this equation:
CH4+NH3+O2→HCN+H2O. A student has 8 g of methane and 10 g of ammonia in excess oxygen.
a. What is the balanced equation for this reaction?
b. Which reagent is limiting? Explain why.
c. How many grams of hydrogen cyanide will be formed?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 05:50
What happens when the temperature of a solution increases?
Answers: 2

Chemistry, 23.06.2019 00:30
There are approximately 15 milliliters (ml) in 1 tablespoon (tbsp). what expression can be used to find the approximate number of milliliters in 3 tbsp?
Answers: 1

Chemistry, 23.06.2019 15:40
The table below shows the freezing points of four substances. substance freezing point (°c) benzene 5.5 water 0 butane –138 nitrogen –210 the substances are placed in separate containers at room temperature, and each container is gradually cooled. which of these substances will solidify before the temperature reaches 0°c? benzene water butane nitrogen
Answers: 2

Chemistry, 23.06.2019 19:10
Which of the following questions can use science as a method of inquiry? is cloning appropriate? how can we protect crops from drought? why is jazz a great type of music? did dinosaurs exist?
Answers: 2
You know the right answer?
Methane (CH4), ammonia (NH3), and oxygen (O2) can react to form hydrogen cyanide (HCN) and water acc...
Questions

Mathematics, 01.02.2021 18:20

Mathematics, 01.02.2021 18:20

Mathematics, 01.02.2021 18:20


Spanish, 01.02.2021 18:20

Mathematics, 01.02.2021 18:20

Biology, 01.02.2021 18:20

Physics, 01.02.2021 18:20



Biology, 01.02.2021 18:20


Physics, 01.02.2021 18:20

Mathematics, 01.02.2021 18:20




Mathematics, 01.02.2021 18:30

History, 01.02.2021 18:30

Mathematics, 01.02.2021 18:30

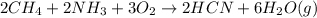
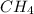 is the limiting.
is the limiting.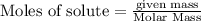
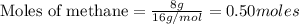
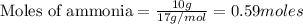
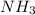
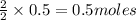 of
of